Are new oral anti-COVID-19 drugs effective?
What the research says:
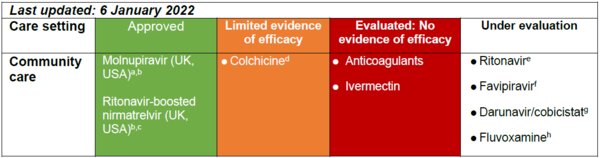
The oral antiviral agents molnupiravir and ritonavir-boosted nirmatrelvir (Paxlovid) are approved for use in the UK (MHRA, 4 November 2021; MHRA, 31 December 2021), and both agents have been granted emergency use authorisation for use in the USA (CDC 2021), in patients with mild to moderate COVID-19 who are at the beginning of symptom onset and at higher risk of progression to severe disease. Systemic oral steroid treatment (dexamethasone, prednisolone, methylprednisolone, hydrocortisone) is recommended for in-hospital or advanced care at home of patients with COVID-19 illness (WHO 2020). Evidence is emerging that high-dose oral steroids may reduce the risk for severe hypoxia in COVID-19 illness (WHO REACT Working Group 2020). Oral agents approved, with limited evidence of efficacy and that are under evaluation for the treatment of patients with COVID-19 in the community care setting are shown in the Table below:
aMHRA. Regulatory approval of Lagevrio (molnupiravir). Available at: https://www.gov.uk/government/publications/regulatory-approval-of-lagevrio-molnupiravir. Published: 4 November 2021; bCDC. The COVID-19 Treatment Guidelines Panel’s statement on therapies for high-risk, nonhospitalized patients with mild to moderate COVID-19. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-paxlovid-drug-drug-interactions/. Published 30 December 2021; cMHRA. Regulatory approval of Paxlovid. Available at: https://www.gov.uk/government/publications/regulatory-approval-of-paxlovid. Published: 31 December 2021; dTardiff JC, et al. Pre-print, not yet peer reviewed, available at: https://www.medrxiv.org/content/10.1101/2021.01.26.21250494v1; ePfizer Press Release. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate. See also Owen et al 2021; fApproved for the treatment of influenza and currently under investigation in the PRINCIPLE Study; gApproved for the treatment of HIV infection and currently under investigation in the PRINCIPLE Study; hReis et al 2021.
What this means for your clinical practice:
- The oral antiviral agents molnupiravir and ritonavir-boosted nirmatrelvir (Paxlovid) are approved for use in the UK and both have been granted emergency use authorisation* for use in the USA. There are currently no other guideline-recommended oral treatments for the management of the acute COVID-19 infection in the community setting although other treatments are approved for use in this setting.
- Medicinal treatment of acute COVID-19 should currently focus on symptomatic relief.
- Antipyretics, paracetamol or ibuprofen, can be used for the management of fever and pain, there is no evidence to suggest they halt progression to more severe disease.
- Diarrhoea and nausea should be managed according to usual standard of care.
- Antibiotic prophylaxis is not advised and antibiotics should not be prescribed unless there is a clinical suspicion of a bacterial infection, the risk of which has been shown to be low.
- A number of new oral drugs are currently under investigation including antiviral agents, and fluvoxamine for the treatment of patients with COVID-19 in the community setting.
*An Emergency Use Authorization permits the use of a medical product that has either not yet received regulatory approval or has not received regulatory approval for a specific indication, to be used in an emergency situation to diagnose, treat, or prevent serious or life-threatening diseases or conditions
Useful links and supporting references:
CDC. The COVID-19 Treatment Guidelines Panel’s statement on therapies for high-risk, nonhospitalized patients with mild to moderate COVID-19. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-paxlovid-drug-drug-interactions/. Accessed January 2022.
Merck and Ridgeback’s investigational oral antiviral molnupiravir reduced the risk of hospitalization or death by approximately 50 percent compared to placebo for patients with mild or moderate COVID-19 in positive interim analysis of Phase 3 study. Available at: https://www.merck.com/news/merck-and-ridgebacks-investigational-oral-antiviral-molnupiravir-reduced-the-risk-of-hospitalization-or-death-by-approximately-50-percent-compared-to-placebo-for-patients-with-mild-or-moderat/. Accessed December 2021.
MHRA. Regulatory approval of Lagevrio (molnupiravir). Published: 4 November 2021. Available at: https://www.gov.uk/government/publications/regulatory-approval-of-lagevrio-molnupiravir. Accessed January 2022.
MHRA. Regulatory approval of Paxlovid. Published: 31 December 2021. Available at: https://www.gov.uk/government/publications/regulatory-approval-of-paxlovid. Accessed January 2022.
Owen DR, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Pre-print, not yet peer reviewed, available at: https://www.medrxiv.org/content/10.1101/2021.07.28.21261232v1. Accessed December 2021.
Popp M, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database of Systematic Reviews 2021:CD015017. Available at: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD015017.pub2/full. Accessed December 2021.
PRINCIPLE study in community patients: https://www.principletrial.org/. Accessed December 2021.
Reis G, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2021;S2214-109X(21)00448-4. Available at: https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(21)00448-4/fulltext. Accessed December 2021.
Tardiff JC, et al. Efficacy of colchicine in non-hospitalized patients with COVID-19. Pre-print, not yet peer reviewed, available at: https://www.medrxiv.org/content/10.1101/2021.01.26.21250494v1. Accessed December 2021.
World Health Organization. A living WHO guideline on drugs for covid-19. Available at: https://www.bmj.com/content/370/bmj.m3379. Accessed December 2021.
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19. A meta-analysis. JAMA 2020;324:1330–41. Available at: https://jamanetwork.com/journals/jama/fullarticle/2770279. Accessed November 2021.
Authors:
Tiago Maricoto, MD, PhD (Family Doctor, Aradas Health Unit and University of Beira Interior, CACB-Clinical Academic Centre of Beiras, Portugal) for and on behalf of the IPCRG practice driven answers review group.
Resource information
- COVID-19
- Vaccination