What medicines are available to treat an acutely unwell person with COVID-19 in the primary care setting to reduce the risk of hospital admission?
This question will be reassessed on a regular basis due to the fast changing evidence
What the research says:
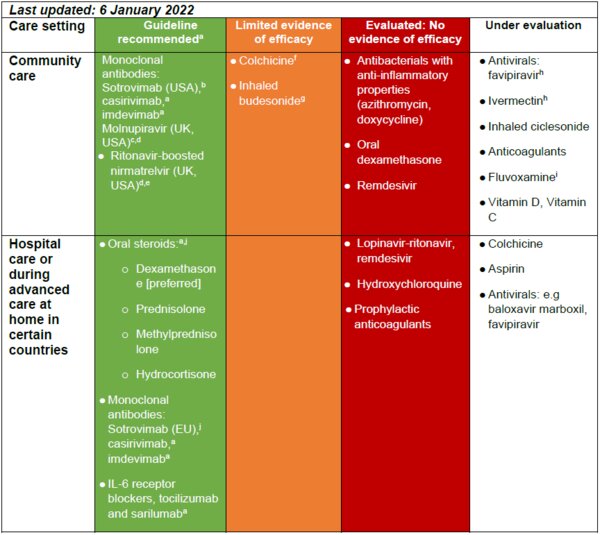
The monoclonal antibodies casirivimab and imdevimab are recommended by the WHO as a one off dose (subcutaneous or intravenous) for patients with non-severe COVID-19 at the highest risk of hospitalisation (WHO 2021). In the USA, the monoclonal antibody sotrovimab has been granted emergency authorisation* for use in the community setting for the treatment of children aged >12 years and adults with mild-to-moderate COVID-19 illness who are at risk for severe disease (GSK 2021). The oral antiviral agents molnupiravir and ritonavir-boosted nirmatrelvir (Paxlovid) are approved for use in the UK (MHRA, 4 November 2021; MHRA, 31 December 2021), and both agents have been granted emergency use authorisation for use in the USA (CDC 2021), in patients with mild to moderate COVID-19 who are at the beginning of symptom onset and at higher risk of progression to severe disease. A range of medicines are under evaluation for use in primary care with a view to i) symptom relief/control, ii) reducing the likelihood of hospitalisation iii) reducing the need for intensive care unit care and of death.
*An Emergency Use Authorization permits the use of a medical product that has either not yet received regulatory approval or has not received regulatory approval for a specific indication, to be used in an emergency situation to diagnose, treat, or prevent serious or life-threatening diseases or conditions
aWorld Health Organization. Therapeutics and COVID-19: living guideline. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.1.
bApproved by the US FDA for Emergency use for the treatment of mild-to-moderate COVID-19 in adults and children >12 years at risk for progression to severe COVIDI-19. Available at: https://www.sotrovimab.com/.
cMHRA. Regulatory approval of Lagevrio (molnupiravir). Available at: https://www.gov.uk/government/publications/regulatory-approval-of-lagevrio-molnupiravir. Published: 4 November 2021.
dCDC. The COVID-19 Treatment Guidelines Panel’s statement on therapies for high-risk, nonhospitalized patients with mild to moderate COVID-19. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-paxlovid-drug-drug-interactions/. Published 30 December 2021.
eMHRA. Regulatory approval of Paxlovid. Available at: https://www.gov.uk/government/publications/regulatory-approval-of-paxlovid. Published: 31 December 2021.
fTardiff JC, et al. Pre-print, not yet peer reviewed, available at: https://www.medrxiv.org/content/10.1101/2021.01.26.21250494v1.
gSTOIC study. Available at: https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(21)00160-0/fulltext.
hPRINCIPLE study in community patients: https://www.principletrial.org/.
i Reis et al. Lancet Glob Health 2021;S2214-109X(21)00448-4.
jApproved by the European Medicines Association for IV administration for patients aged >12 years with COVID-19 who do not require supplemental oxygen but are at risk for progression to severe COVID-19: https://www.ema.europa.eu/en/documents/referral/sotrovimab-also-known-vir-7831-gsk4182136-covid19-article-53-procedure-conditions-use-conditions_en.pdf. See also Gupta A, et al. Preprint available at: https://doi.org/10.1101/2021.05.27.21257096.
Although no specific studies have been conducted on the efficacy and safety of paracetamol it remains widely used as part of a standard of care for symptoms of COVID-19, with no reports of safety concerns identified. Similarly, for ibuprofen, despite initial concerns, no data indicating safety concerns exist in the context of COVID-19 infection.
What this means for your clinical practice:
- The monoclonal antibodies casirivimab and imdevimab are recommended by the WHO as a one off dose (subcutaneous or intravenous) for patients with non-severe COVID-19 at the highest risk of hospitalisation.
- In the USA, following FDA emergency authorisation, sotrovimab may be considered for the treatment of children aged >12 years and adults with mild-to-moderate COVID-19 illness who are at risk for severe disease (GSK 2021).
- The oral antiviral agents molnupiravir and ritonavir-boosted nirmatrelvir (Paxlovid) are approved for use in the UK and both have been granted emergency use authorisation for use in the USA.
- Medicinal treatment of acute COVID-19 should currently focus on symptomatic relief.
- Standard antipyretics, paracetamol or ibuprofen, can be used for the management of fever and pain, there is no evidence to suggest they halt progression to more severe disease.
- Diarrhoea and nausea should be managed according to local standard of care.
- Antibiotic stewardship remains important during the COVID-19 pandemic. Antibiotic prophylaxis is not advised and antibiotics should not be prescribed unless there is a clinical suspicion of a bacterial infection
- At the present time there is no evidence to support the use of oral steroids or prophylactic oral anticoagulants for the treatment of patients with COVID-19 in the community setting.
- While a phase 2 study in 146 patients has shown inhaled budesonide used within 7 days of COVID-19 symptom onset reduced the need for hospitalisation, larger studies are ongoing to determine whether this agent is appropriate for routine use in the primary care setting.
- For more information on the emerging data for the use of inhaled corticosteroids in the community management of acute COVID-19 please see here.
- For more information on oral COVID treatments please see here.
With grateful thanks to Professor Janwillem Kocks (President, IPCRG) for and on behalf of the IPCRG practice driven answers review group.
Resource information
- COVID-19
- Disease management
- Treatment - drug
- Treatment - non-drug