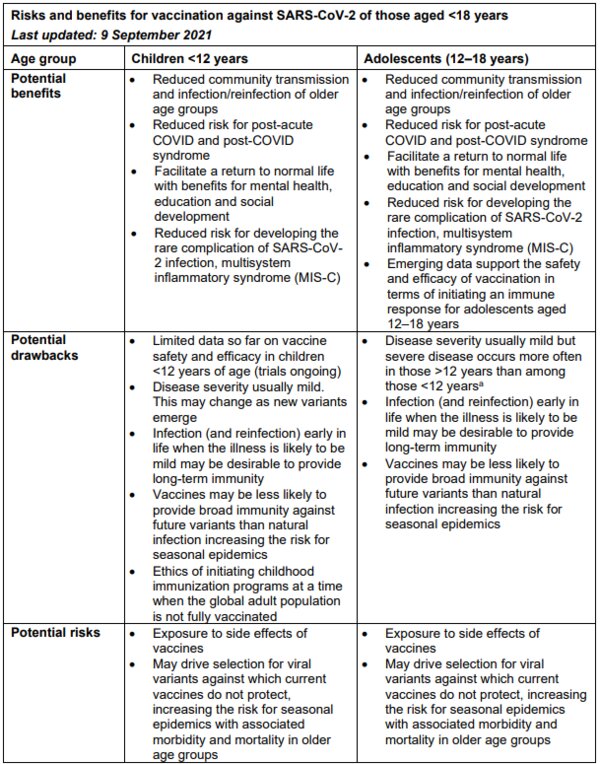
What are the potential benefits and risks for vaccination of children and adolescents against SARS-CoV-2?
What the research says:
The safety and effectiveness of vaccines against the SARS-CoV-2 virus have been evaluated in healthy children and adolescents. The Pfizer-BioNTech vaccine has been evaluated as suitable for use in children >12 years by the World Health Organization and has emergency use approval from the FDA for use in the US for children and adolescents. This vaccine is already being used in a number of EU and non-EU countries to vaccinate children >12 years of age. The Moderna vaccine is also now approved for use in those > 12 years old in Canada. The results of the CoronaVac study indicates that vaccination is safe and induces immune responses in Chinese children and adolescents aged 3–17 years (Han et al 2021). As of August 2021, 18 phase 1 to 3 studies of various SARS-CoV-2 vaccines are listed in clinicaltrials.gov in children and adolescents with completion dates ranging from 2021 to 2025.
Children <12 years: For the majority of healthy children <12 years of age COVID-19 is a mild disease. This may change as data emerges on the effect of new variants on disease severity in children. The justification for vaccinating children <12 years of age will likely require evidence for reduced transmission. However, the contribution of children as a vector for transmission of the virus through the population remains unclear, with conflicting results from epidemiologic trials.
Adolescents (12–18 years): Data are emerging to indicate that adolescents are at increased risk for severe COVID-19 disease, hospitalization and death compared with children <12 years of age (CDC 2021). Many countries have now initiated vaccination programmes for healthy adolescents >12 years of age.
aData from COVID-NET in the US: https://www.cdc.gov/mmwr/volumes/70/wr/mm7023e1.htm
Children and adolescents with underlying conditions that might place them at increased risk for more severe disease may benefit most at an individual level from SARS-CoV-2 vaccination. Risk factors for more severe COVID-19 illness in children include immunocompromise, pulmonary disease, diabetes, obesity, renal disease, underlying neurological conditions and learning disabilities (Graff et al 2021).
What this means for your clinical practice:
• Parents with concerns regarding vaccination for their children, or adolescents with concerns, can be advised that the data suggest the vaccines available for use in those aged 12-18 years of age are safe and any side effects likely to be experienced are mild and similar to those we might expect with other vaccines
• The evidence suggests the risk of serious side effects is small, but more data will emerge on this as the pandemic evolves and vaccination continues
• Should ongoing surveillance indicate that new SARS-CoV-2 variants cause more severe illness in those aged <18 years of age, the benefits of vaccination may outweigh associated risks and re-evaluation will be required
• SARS-CoV-2 vaccine programs should be initiated and delivered according to National guidelines, with vaccines used according to their licenses
• National guidelines should be followed when determining whether a child is at risk for more severe COVID-19 illness and is therefore eligible for SARS-CoV-2 vaccination
Useful links and supporting references:
CDC. Hospitalization of adolescents aged 12–17 years with laboratory confirmed COVID-19 – COVID.NIT, 14 States, March 1, 2020–April 24, 2021. MMWR 2021:70;851–857. Available at: https://www.cdc.gov/mmwr/volumes/70/wr/mm7023e1.htm. Accessed September 2021.
Graff K, et al. Risk factors for severe COVID-19 in children. Ped Infect Dis J 2021;40:e137-e145. Available at: https://doi.org/10.1097/inf.0000000000003043. Accessed September 2021.
Han B, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a randomised, double-blind, and placebo-controlled, phase 1/2 clinical trials. Lancet Infect Dis 2021. Available at: https://doi.org/10.1016/S1473-3099(21)00319-4. Accessed September 2021.
Authors:
Dr Fiona Mosgrove (GP and Clinical Lead Grampian Respiratory Improvement Programme, Aberdeen, Scotland, UK) for and on behalf of the IPCRG practice driven answers review group.
Resource information
- COVID-19
- Children
- Vaccination